By: George Mathew
Key takeaways
- The pharmaceutical industry is facing several challenges such as price fluctuations in APIs, dependency on imports, and shortages of raw materials, which are affecting the availability and prices of the end-products.
- To mitigate the risks and ensure reliability, API contracts must contain specific clauses that include mechanisms for price adjustments and contingency plans for supply disruptions.
- Collaborative relationships with suppliers can help in reducing costs and ensuring a stable supply of APIs, which can benefit the entire pharmaceutical industry through improved stability and efficiency.
- When selecting API suppliers, it is essential to prioritize factors like quality assurance, regulatory compliance, supply chain resilience, technical expertise, capacity, reliability, cost, intellectual property protection, sustainability, and effective communication.
The Active Pharmaceutical Ingredient (API) industry has recently grown remarkably, driven by various factors. One of the main reasons for the growth in the pharmaceutical industry is the rise in chronic diseases and the growing global population. This has resulted in a significant demand for affordable and effective medicines. Therefore, pharmaceutical companies are focusing on developing innovative Active Pharmaceutical Ingredients (APIs) that can be used in the manufacturing of various pharmaceuticals. Furthermore, advancements in technology and manufacturing techniques have contributed to improved API quality and cost-effectiveness, thereby fueling their market expansion.
What is the major concern?
There is a growing global concern about the shortage of medicines. These shortages have multifaceted causes that are deeply rooted in the complex interdependence of the global pharmaceutical industry. Some of the factors that contribute to these shortages include unexpected and temporary spikes in demand, production challenges related to quality, supply chain disruptions, and transportation issues.
Each and every action that pharmaceutical businesses do during the medicine production process is vital, but the addition of APIs is very important. Large pharmaceutical companies frequently rely on vendors to provide these necessary substances and the intermediate parts of them. Assessing suppliers in the global API market is vital to ensure the quality, reliability, and compliance of the raw materials utilized in drug manufacturing.
Niche Active Pharmaceutical Ingredients (APIs) play a pivotal role in this landscape. These specialized APIs cater to specific therapeutic areas, such as anti-infective APIs, addressing the unique needs of patients with particular medical conditions. Leading pharmaceutical organizations, including pharmaceutical giants and smaller niche API providers, rely on top API manufacturers like LGM Pharma to source and synthesise high-quality APIs and intermediates. Effective storage of APIs is also essential to maintain their integrity and efficacy throughout the manufacturing process.
To navigate the complexities of the API industry, pharmaceutical companies often enlist the support of pharmaceutical sourcing firms or procurement pharmaceutical companies. These entities assist in sourcing reliable API suppliers, ensuring adherence to API regulation and compliance standards, and optimizing the overall supply chain efficiency. By forming strategic alliances and carefully vetting suppliers, pharmaceutical companies may reduce risks and guarantee a consistent supply of premium APIs for the production of drugs.
Challenges for fostering innovation in pharmaceutical sourcing and procurement:
API price changes and raw material shortages impact end-product prices
Challenges in sourcing APIs, including price fluctuations, import dependency, and raw material shortages, have direct implications on pharmaceutical manufacturing. The pandemic and Ukraine war disrupted the global supply chain, increasing transportation costs and raw material expenses, affecting pharmaceutical ingredients and packaging. Approximately 70% of APIs are imported into the US. China accounts for roughly 10%–15% of the U.S. API market, while the EU, amid the Russia-Ukraine conflict, providing 20%–25% of API manufacturing facilities, leading to supply chain disruptions and raw material shortages. Effective mitigation strategies and resilient supply chain management are crucial to ensure consistent and affordable pharmaceutical product availability.
API contracts require specific clauses to mitigate risks and ensure reliability
In contracts pertaining to Active Pharmaceutical Ingredients (APIs), addressing challenges is paramount. Including specific clauses is vital to mitigate risks and ensure the consistency of supply, product quality, and pricing. Such clauses can encompass price adjustment mechanisms for market fluctuations, stringent quality control standards, and contingency plans for supply disruptions. By incorporating these provisions, pharmaceutical companies can effectively navigate API-related challenges, maintaining a reliable and cost-effective supply chain.
Supplier collaboration saves costs and ensures a stable supply of APIs

Effective handling of challenges related to Active Pharmaceutical Ingredients (APIs) can be achieved through collaborative supplier relationships. Pharmaceutical companies can efficiently manage input costs and secure lower raw material prices by partnering with suppliers. This collaboration improves supply chain stability, cost efficiency, and ensures a dependable API source, ultimately benefiting the entire pharmaceutical industry.
Important factors to consider during the niche API sourcing process
When selecting suppliers for pharmaceutical ingredients, several factors should be considered. Here are some of the main ones:
Quality Assurance:
Prioritize suppliers who have strong quality control measures to ensure that the active pharmaceutical ingredients (APIs) are consistently pure and meet the required standards.
Regulatory Compliance:
Ensure that suppliers comply with regulatory standards like Good Manufacturing Practices (GMP) and have the necessary certifications from organizations like the FDA or EMA.
Supply Chain Resilience:
Assess suppliers’ ability to maintain a stable and resilient supply chain, which includes backup plans for raw material sourcing and production interruptions.
Technical Expertise:
Choose suppliers who have experience in producing similar compounds and have a proven track record of expertise in niche API synthesis and manufacturing processes.
Capacity and Scalability:
Evaluate suppliers’ production capacity to meet current and future demand requirements, taking into account scalability and flexibility in production volumes.
Reliability and Reputation:
Look for suppliers with a reputation for reliability, on-time delivery, and responsiveness to customer needs, backed by positive references and reviews.
Cost and Pricing:
Consider cost factors, such as raw material costs, manufacturing processes, and total landed costs, while balancing them with quality and reliability.
Intellectual Property Protection:
Ensure that suppliers have appropriate measures in place to protect intellectual property rights related to the API synthesis process and proprietary formulations.
Sustainability and Environmental Impact:
Consider suppliers’ commitment to sustainability practices, including waste management, energy efficiency, and environmental impact reduction.
Communication and Collaboration:
Establish clear communication channels and foster collaborative relationships with suppliers to facilitate transparency, feedback, and continuous improvement throughout the sourcing process.
How SpendEdge can help to tackle challenges
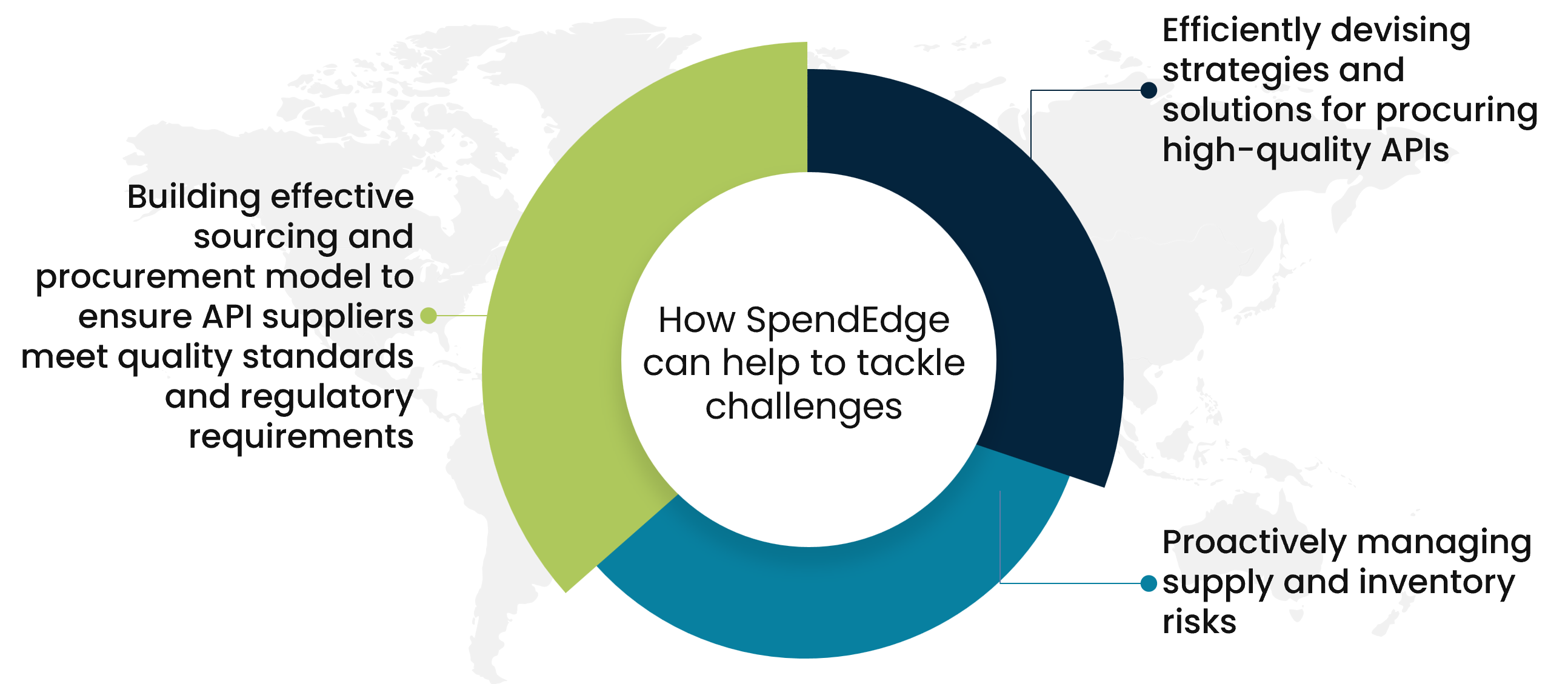
Building effective sourcing and procurement model to ensure API suppliers meet quality standards and regulatory requirements
Sourcing advisors are essential in the API sourcing and procurement process. Our experts assist in supplier identification, evaluate supplier capabilities, ensure compliance with quality requirements, and conduct supplier audits to adhere to cGMP and global regulations. Our specialist advisors play a crucial role in maintaining pharmaceutical supply chains’ integrity and quality standards.
Efficiently devising strategies and solutions for procuring high-quality APIs
Our advisors help clients in effectively source high-quality APIs, ensure regulatory compliance, and promote sustainability in pharmaceutical contract manufacturing. Our experts conduct regular sustainability audits of API suppliers to ensure they are adhering to sustainable practices and continuously improving their environmental performance.
Proactively managing supply and inventory risks
Our experienced advisors helping pharmaceutical companies implement Low-Cost Country Sourcing (LCCS) or Best Cost Country Sourcing (BCCS) strategies for API procurement. Our experts offers invaluable insights to assist in pinpointing the optimal countries for sourcing APIs. They meticulously assess multiple variables, including production expenses, transportation costs, product quality, labor availability, and geographical proximity, to determine the most fitting sourcing destinations for the organization’s medicinal products. These analyses empower informed decision-making and the formulation of cost-efficient procurement strategies.
Success story: How SpendEdge helped a pharmaceutical company to mitigate delayed API deliveries and regulatory issues
Our client is a German-based prominent pharmaceutical company, known for producing a range of life-saving medications.
The client has experienced consistent delays in API deliveries from multiple suppliers and regulatory scrutiny due to non-compliance issues related to some of their API suppliers. These delays and regulatory challenges disrupted the company’s production schedules, leading to product shortages and potential revenue losses.
Our experts helped the client in assessing and analyzing potential API suppliers and onboard alternative API suppliers with a history of reliable deliveries. Our advisors conducted regular supplier audits to identify and address compliance issues proactively to ensure adherence to international quality standards and regulatory requirements.
The solution offered by our experts helped the client successfully mitigate the challenges related to delayed API deliveries and regulatory issues, which bolstered the company’s reputation and market position.
Conclusion
In conclusion, the pharmaceutical industry‘s growth, particularly in the realm of Active Pharmaceutical Ingredients (APIs), is driven by a multitude of factors, including the increasing prevalence of chronic diseases and the expanding global population. This surge in demand necessitates the development of innovative APIs, such as anti-infective APIs, to cater to specific therapeutic needs. Leading pharmaceutical organizations, ranging from pharmaceutical giants to niche API providers, rely on top manufacturers like LGM Pharma to source high-quality APIs and intermediates, ensuring the integrity and efficacy of their products. To address these challenges, pharmaceutical companies enlist the support of pharmaceutical sourcing firms and procurement pharmaceutical companies to navigate the complexities of the API industry. These entities assist in sourcing reliable API suppliers, ensuring compliance with API regulation and quality standards, and optimizing supply chain efficiency. By prioritizing factors such as quality assurance, regulatory compliance, and supply chain resilience, pharmaceutical companies can ensure a stable and cost-effective supply of APIs for drug manufacturing.

Contact us now to solve your procurement problems!
Author’s Details
Arthi,
Associate Vice President, Sourcing and Procurement Intelligence
Arthi is an Associate Vice-President with Infiniti Research. She leads one of the procurement intelligence groups within the organization supporting global life sciences and FMCG companies by proving data-driven procurement insights.




