By: Srinivas R
Key Takeaways:
- Advancements in medical technology: Digital health solutions, telemedicine, and AI-driven diagnostics are transforming patient care and sustainability efforts in the healthcare industry.
- ESG Integration: MedTech companies and healthcare providers must embed ESG principles throughout the MedTech value chain to meet regulatory standards and build consumer trust.
- Supply Chain Sustainability: Ethical sourcing and optimizing supply chains are crucial for the pharmaceutical and medical device industries to align with ESG standards.
- Stakeholder Expectations: Transparency and proactive ESG measures enhance relationships with stakeholders, ensuring better patient care and long-term resilience in the healthcare sector.
Businesses across industries, especially in developed economies, risk getting entangled in a complex web of environmental, social, and governance (ESG) legislations unless they are nimble and agile enough to address them. Time is of the essence here, and the cost of compliance could exceed a tenth of the sales revenue of medical device companies (medtechs) in some instances at least. Today medtechs are called upon to report their performance on multiple fronts to a plethora of regulatory bodies such as the SEC (financials), EPA (environmental impact), OSHA (workforce wellbeing and safety) and FDA (device safety and efficacy). Outside of these, there are a number of ESG standards medtechs can embrace of their own accord (e.g., SASB, GRI, PRI). Let’s face it, with so much in-depth and revealing data from across operations in the hands of stakeholders, there is virtually nothing or little any business, leave alone medtechs, can hope to conceal. Businesses are in the spotlight and their data is in full public view, while citizens, lawmakers, non-profits, and the media are ranking them based on their likelihood of tackling climate change, social inequity, and unethical practices. Being more transparent is not just chic, but can be more paying for businesses, as many early ESG adopters have already realized, than opaque corporate practices that can at best breed stakeholder mistrust. Medtechs with enhanced ESG ratings have warmer relationships with stakeholders, consistently track their environmental footprint, tend to be asset-wise stronger in their balance sheets, and court fewer controversies. Studies indicate businesses ranked high on ESG are more likely to weather market downturns and attract lower-cost capital. ESG score will also increasingly matter as a key procurement criterion in the days ahead.
Why ESG Matters for MedTech
With the dawn of a new era in the MedTech sector, it is no longer just a trend but a necessity to include ESG principles. Fast progress in medical technology is anticipated in the next ten years, along with a greater emphasis on sustainability and moral leadership. Innovations that address medical requirements while giving priority to social and environmental considerations are what the sector may anticipate. By decreasing reliance on physical infrastructure and transportation, the rise of digital health solutions, telemedicine, and AI-driven diagnostics will revolutionise patient care and lower the carbon footprint.
Some ESG measures that are gaining currency in the medtech space include reprocessing single-use devices and plastics, use of more biodegradable materials (like bamboo procedure trays), and sustainable product packaging and recycling. Adopting digital and telehealth services is also high on the agenda of many medtechs seeking to improve care for impoverished communities who are at the most risk of falling through the cracks of an unequal healthcare system. More than ever before, medtechs are paying attention to diversifying their workforce and ensuring more inclusivity. Many medtechs are also working overtime to reduce emissions from direct operations in fulfillment of the 2040 vision of net-zero carbon emissions. Of course, nothing is enough and there are no full stops when it comes to ESG action…
Businesses and regions often hold differing ESG perceptions
Putting ESG precepts into practice is not as easy in the medtech sector as is popularly imagined. The expanding scope of the ESG discipline is keeping the medtech C-suites befuddled. This is not surprising since the medical devices industry has an expanding gamut of issues before it to address in the name of ESG, ranging from transparent financial practices, labor codes, and workforce wellbeing to air quality and data security. The list is almost endless and includes diversity, equity, and inclusion as well as designing products for circularity, thus minimizing waste and maximizing reuse and recyclability. Regardless of region, there is some broad agreement around the end goals of ESG, namely, substantial reduction in greenhouse gas reductions by 2030, adoption of cleaner energy sources, improving workplace diversity, and ensuring all stakeholders have access to a medtech’s financial information. However, when it comes to near-term ESG initiatives, what’s good for the goose is not necessarily good for the gander. Diversity, income equality, workplace injury rates, philanthropy, and labor practices of suppliers figure large on the radars of North American CXOs. Cut to Western Europe, the immediate focus there is likely to be on the environmental impact of medtechs and their supply chain participants.
The pace of regulatory expansion is leaving businesses breathless
Procurement teams in the medical devices business face the unenviable task of keeping up with shifting goalposts as lawmakers up the game in terms of ESG disclosure legislations. The sheer number of regulations that have come into effect in recent times and their terms of reference can be overpowering, especially for smaller medical device manufacturers. For instance, the EPA is responsible for stewarding efforts by medtechs to reduce the environmental footprint of their input materials and production process as well as cut back energy wastage. But there are valid concerns that even a few of the too-stringent proposals by the EPA might make business very nearly unsustainable for SMEs and legacy players in the medtech landscape while cutting off accessible medical care for some of the most needy patients.
The cost of compliance can be high
Cost and time are of the essence in the majority of ESG implementations for medical device manufacturers. For instance, take the case of ethylene oxide (EtO) reduction. Almost half of the 40 billion-plus medical devices in the US are sterilized every year using this clear gas. For materials sensitive to heat or moisture, this is the only effective sterilization technique. However, amid concerns about the carcinogenic effect of prolonged exposure to EtO on workers at commercial sterilization facilities and people in their vicinity, including school children, EPA proposed this April that sterilizers install pollution controls within 18 months to cut back EtO emissions by four-fifths every year. The cost of regulatory compliance in this case is expected to cross $200 million annually by way of capex and opex. This could have more consequences than just putting the budgets of SMEs in the US medical devices market under strain. For two out of every five commercial sterilizer firms, regulatory compliance costs are going to be greater than 10% of their sales revenue. It’s common knowledge that where regulatory costs overshoot company revenue by more than 3%, that’s a sure-shot recipe for business failure, something even federal agencies acknowledge. Cost overruns put small operators on slippery turf more than anyone else. Most importantly, of the nearly 7,000 participants in the $56 billion market, the majority are SMEs already weighed down by slim sales growth. More than four-fifths of them have fifty or fewer employees. Sure enough, the pass-through of compliance costs by medtechs will increase the payment burden for healthcare providers and end users, leading to treatment dropout by patients. Paradoxically, this could be a setback for the social agenda or the “S” in ESG whose professed aim is to ensure products are accessible and affordable to all patients!
Ensuring vendor compliance is a tough call for many medtechs
Since any supply chain is only as strong as its weakest link, businesses must strive to bring their suppliers worldwide to speed on all three pillars of ESG. Medtech is an R&D-intensive domain, with the top five companies channeling more than a fifth of their revenue stream into new product innovation. The biggest 100 medtechs have upped their R&D budgets by more than 5% in 2023 over the previous year. To rationalize overall spend, Western medtech OEMs are increasingly outsourcing their medical device production to emerging markets (e.g., Brazil, India, China). Global medical device outsourcing industry stood at $65 billion in 2022. Global markets present a pandora’s box of opportunities medtechs must crowbar open. Deep talent pools and inherent capabilities for frugal innovation are key strengths of globalized supply chains. However, without deep insights into the environmental, social, and governance practices of supply chain participants, medtech might be setting the clock back on the sustainability front. It might be expeditious to go with a vendor with the lowest quote, but not for too long. Without developing comprehensive ESG scores of suppliers by keying in granular data from environmental, social, labor, governance, and ethical practices, businesses risk getting caught in the crosshairs of overzealous regulators in the not too long run. An ultra-careful vetting of suppliers is a must for any medtech enterprise worth its name.
Key technology trends impacting esg performance
Advancements in medical technology are significantly shaping the landscape of Environmental, Social, and Governance (ESG) performance in the healthcare industry. As sustainability becomes a focal point, the integration of innovative solutions is vital for meeting evolving medical needs and enhancing patient care.
Digital health solutions and telemedicine
The rise of digital health solutions and telemedicine has transformed how healthcare providers deliver services. These technologies improve access to care, especially in remote areas, reducing the environmental impact associated with travel. Additionally, telemedicine supports social aspects of ESG by ensuring that more patients receive timely and efficient care.
AI-driven diagnostics
AI-driven diagnostics are revolutionizing the pharmaceutical industry and medical device industry. These technologies enable more accurate and early disease detection, which enhances patient care outcomes and reduces the environmental burden of prolonged treatments. AI applications in clinical trials streamline processes, making them more efficient and sustainable.
Medtech companies and the value chain
Medtech companies are at the forefront of integrating ESG principles into their operations. From research and development to the distribution of products, the entire medtech value chain is being optimized for sustainability. This commitment not only meets regulatory requirements but also builds consumer trust, as patients increasingly prefer environmentally conscious healthcare providers.
Impact on hospitals and healthcare institutions
Hospitals and healthcare institutions are adopting green technologies to reduce their carbon footprint. Innovations such as energy-efficient medical devices and sustainable waste management practices are essential for achieving ESG goals. These advancements also resonate with social responsibility by providing safer and healthier environments for both patients and staff.
Supply chains in the pharmaceutical and medical device businesses
Sustainability in supply chains is a critical aspect of ESG performance. Pharma and medical device businesses are focusing on ethical sourcing of materials, reducing waste, and optimizing logistics. This holistic approach ensures that the entire lifecycle of medical products, from production to disposal, aligns with ESG standards.
How SpendEdge can help with medtech strategies
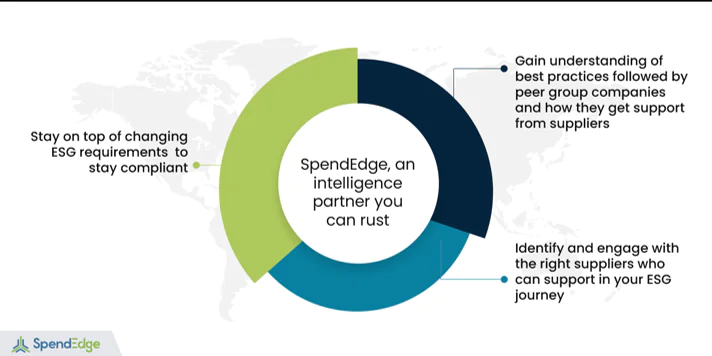
Embed sustainability in the business DNA
Incorporating ESG in their procurement stream is perhaps the only way ahead for enterprises in an era of elevated stakeholder consciousness. At SpendEdge, our specialists help businesses identify the key sustainability initiatives their competitors have embraced to gain significant traction in the market.
Cascade the ESG mantra across the supply chain
Ensuring adherence to the ESG framework is a priority for medtechs seeking to decarbonize operations and improve ESG performance as reflected in the eyes of key stakeholders. At SpendEdge, we help businesses understand the ESG landscape better and implement right practices, select best-fit suppliers and remain compliant. Furthermore, our experts break down their cost implications and suggest what fits best for the client.
Success story: A medical devices major leads the way in key supply-side sustainability metrics
Our client is a leader in medical devices based in North America offering technologies and therapies including implantable electronic devices, robotic surgical systems, insulin pumps, and patient monitoring systems. Until the latest quarter 2023, the client has reported appreciable revenue growth, underpinned by resumption in medical procedures in the US and growth of cardiovascular devices, specialty therapies, and patient monitoring interventions. However, seen through the prism of ESG, the reliability of its supply-side is a key concern for the client, particularly since the supply chain weaves across multiple global regions with their own outlook on ESG as different from the client’s home turf. Regulations around medical devices are tightening, and the client must meet regulatory mandates set down by a plethora of bodies, namely, the EPA, OSHA, and FDA, not to speak of voluntary ESG standards such as those recommended by the SASB. Many peers in the industry have come under regulatory scrutiny, including for improper practices by their suppliers in other regions. ESG regulations tend to be high-level, and developing a grassroots understanding of their implications is often hard for many enterprises. After all, that is not their core competence area. It is even harder to translate ESG ideas into affirmative action and roll these out across a distributed supply base.
In early 2022, our experts in procurement, with deep understanding of the nuanced medical devices industry, began to work with the client to help improve its sustainability road map leading up to the end of the decade. To start with, our experts set about gleaning the sustainability best practices of the client’s peers, not just in medtech but in other sectors as well, which have yielded them shining results in the past. Having developed an in-depth understanding of their sustainability initiatives and goals, our experts proceeded to juxtapose these sustainability best practices with those of our client with a view to discovering and closing the gaps. As a next step, our experts developed risk scores for an already curated list of vendors across multiple parameters, covering all three pillars of ESG. These included factors like water conservation, GHG emissions, inclusive and equitable hiring practices, employee relations and labor rights, fiscal stability as well as bribery and corruption risks. By applying these stringent eligibility terms, our team arrived at a short list of vendors capable of helping the client to turbocharge its sustainability initiatives and live up to the ESG expectations of investors, shareholders, and key stakeholders.
The client is in advanced rounds of negotiation with one of the vendors on our shortlist. Besides, the client has retained our team to provide them with live updates and research insights from a dynamically evolving regulatory landscape and deliver advisory on how to stay ahead of changing legislation.

Contact us now to solve your procurement problems!
Conclusion
The integration of Environmental, Social, and Governance (ESG) principles is rapidly becoming a cornerstone in the healthcare industry, particularly among MedTech companies. Advancements in medical technology, including digital health solutions, telemedicine, and AI-driven diagnostics, are pivotal in addressing medical needs while fostering sustainability. As healthcare providers and hospitals adopt these innovations, the entire MedTech value chain benefits, from pharmaceutical and medical device businesses to clinical trials and supply chains. By enhancing transparency and building consumer trust, MedTech companies can improve patient care and meet the growing expectations of stakeholders. In this evolving landscape, ESG performance is not only a regulatory requirement but also a strategic advantage for healthcare institutions committed to long-term success and ethical leadership.
Author’s Details
Srinivas R
Associate Vice President, Sourcing and Procurement Intelligence
Srinivas is a solution design specialist at Infiniti Research and provides advisory services to clients across the medical devices, pharmaceutical, CPG & FMCG, energy, and ICT sectors. He specializes in the procurement areas of industry benchmarking, cost modeling, rate card benchmarking, negotiation advisory, and supplier intelligence.




